Home
Learn more about the submission process:
Step 1 – Preparing For Your Submission
Step 2 – Creating and Submitting Your Proposal
Step 3 – Awaiting the Results of Your Request
Step 4 – Receiving Funding from Roche
Roche Canada is committed to advancing research and creating value for Canadian patients. In line with this commitment, Roche supports medical and educational activities organized by healthcare organizations/entities and by patient organizations to enhance and maintain patient care and advance research.
Roche also supports the community by providing sponsorship for translational, clinical, and healthcare-related education events including conferences, workshops and philanthropic donations. Roche does not accept requests from contract fundraisers.
Learn more about these funding opportunities, including:
Roche is committed to funding high-quality medical research and education initiatives that benefit the healthcare community and aim to improve patient outcomes in Canada. This includes requests for independent medical research projects, independent education initiatives or healthcare fellowships where there is an unmet medical need aligned to Roche’s scientific strategy. In addition, Roche occasionally has active calls for independent medical education grants.
Medical research grants may provide support for scientific, translational or clinical research activities that are not focused on a therapeutic molecule or Roche product. Requests for project funding involving therapeutic molecules or Roche products should be requested as investigator-initiated studies using the IIS Portal rather than the Roche Canada Funding Request Tool.
All requests for medical research and education support require a detailed description and rationale for the initiative, a detailed budget breakdown, description of the target audience as well as project proposal that may include background, research objectives, knowledge/data gaps addressed, impact on improving patient care, expected outcomes and/or plans for dissemination of outcomes. The request must be made on behalf of a Healthcare Organization (HCO) such as a private or public hospital, clinic, laboratory, research institution, foundation, managed care organization, universal or other teaching institution or learned society, or through which one or more HCPs provide services; individuals can not request research or educational support.
Roche will occasionally seek independent medical education applications through a Call for Grants process.
Roche is committed to advancing research and creating value for Canadian patients. We recognize that to best support patients, we need to effectively engage with patient organizations to ensure their voice and perspective are considered at all times. A Healthcare-Related Entity (HCE) including privately owned organizations that provide medical education to HCPs, patient organizations, professional congress organizers and publishers may request support from Roche for research and education initiatives. Roche cannot support HCE’s that are controlled or owned by an individual stakeholder or their family member, legal representative, or agent.
Requests should include a detailed description and rationale for the initiative, a detailed budget breakdown, description of the target audience as well as project proposal that may include background, research objectives, knowledge/data gaps addressed, impact on improving patient care, expected outcomes and/or plans for dissemination of outcomes.
Giving back to the community is an essential part of Roche’s partnering philosophy. Roche provides sponsorship support for translational, clinical, and healthcare-related education events, workshops and conferences. These requests may or may not align to one of Roche’s therapeutic areas of interest and may be requested on behalf of either a Healthcare-Related Entity or Healthcare Organization. We encourage you to speak with your Roche representative prior to submission if you have questions regarding sponsorship eligibility.
Requests for sponsorship should include a detailed description and rationale for the initiative, description of the target audience, events dates, location and anticipated audience numbers, agenda or draft agenda, sponsorship package and/or letter of request including details on the tangible benefits to Roche. The request should also include a detailed cost breakdown of how sponsorship funds will be used. For example, room rental, catering, speaker fees, etc., as well as details related to other funders involved in supporting the event.
Roche’s philanthropy program aims at making a meaningful impact in our communities through both financial support and employee volunteerism. Our efforts have a broad alignment to our organization as a healthcare company, which allows Roche Canada to consider requests that explicitly align with our approach to philanthropy and may not align with Roche’s therapeutic areas of interest.
Requests for philanthropic support should include a detailed description and rationale for the initiative, description of the target audience, detailed budget breakdown, including details related to other funders’ involvement, and a detailed description of volunteer opportunities. Roche cannot support Healthcare-Related Entities or Healthcare Organizations that are controlled or owned by an individual stakeholder or their family member, legal representative, or agent or businesses that have management or financial ties to a Public Officials or the spouse or dependent children of a Public Official.
Requests for initiatives involving clinical, pre-clinical and/or diagnostic activities that may or may not involve Roche’s therapeutic molecules, are not processed through this Roche Canada Funding Request Tool. Please refer to the Roche Funding Opportunities page for more information.
As with all of Roche’s activities, we strive for high performance and integrity. Roche Canada can only consider initiatives within Canada or those that primarily benefit Canadians and adhere to all guidelines relating to ethical business conduct. We do not evaluate or approve requests based on prescribing habits, drug reimbursement status or decisions, or any specific interactions or relationships that Roche may have with the applicant.
Roche seeks to align support with our commitment to serving patients in areas of high unmet medical need and directs investment towards programs and initiatives that have a direct impact on patient-care within our priority areas of business.
Roche is further committed to supporting brand agnostic medical and educational requests that support healthcare system readiness, novel approaches to patient care, clinical capabilities and capacity as well as increased investment in personalized healthcare and real-world evidence.
Every request received will be evaluated for alignment with Roche’s funding policy, recipient eligibility and adherence to business principles including compliance with both Innovative Medicines Canada (IMC) code and Roche’s business principles. Unfortunately, Roche is not able to support all requests received. In order for requests to be properly evaluated and to ensure that all relevant requirements are met, it is important that the requestor provides full information about the proposed initiative. This includes, but is not limited to, a detailed itemized budget breakdown, description and rationale for the initiative, description and impact on the target audience, letter of request, project proposal and/or sponsorship package.
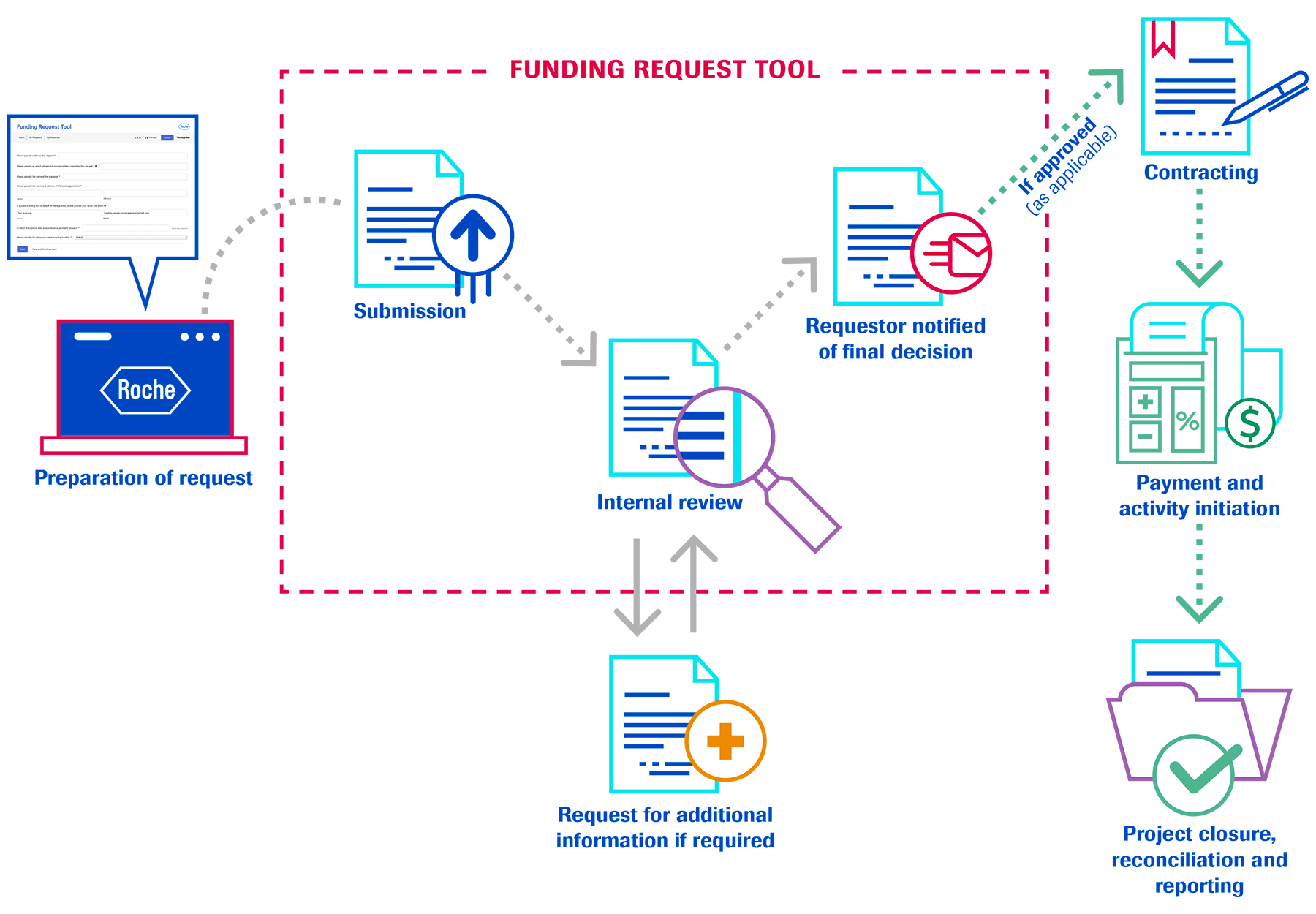
Each request received will be evaluated separately. Previous support by Roche for a similar request does not guarantee future support. Depending on the nature of the request, partial support may be provided. It is recommended that applications are submitted as early as possible to provide Roche sufficient time to fully review your request. All contracting must be in place before the activity begins.
If you have any questions please contact your Roche representative or submit an inquiry using this link.